Developed clinically-actionable gene panels for hearing loss and blindness
Notable proportion of actionable results from high diagnostic yield genomic testing
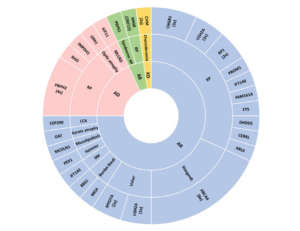 Our Next Generation Sequencing (NGS) gene panels, which include hundreds of genes associated with neurosensorial deafness (OTOgenics™) and hereditary conditions of the retina, choroid and the vitreous and/or optic nerve (OFTALMOgenics™), resulted in an unprecedented diagnostic yield of about 60%.
Our Next Generation Sequencing (NGS) gene panels, which include hundreds of genes associated with neurosensorial deafness (OTOgenics™) and hereditary conditions of the retina, choroid and the vitreous and/or optic nerve (OFTALMOgenics™), resulted in an unprecedented diagnostic yield of about 60%.
Comprehensive genomic testing provided clinically relevant insights in a large proportion of deaf and blind patients, identifying potential therapeutic opportunities or previously undiagnosed syndromes in almost 50% of genetically diagnosed cases. Even more impressive, roughly 25% of the blind patients diagnosed with our NGS technology are candidates for gene therapy clinical trials.
Previous work overseen by Dr. Cabanillas to develop technically challenging molecular tests formed the basis for developing these clinically useful gene panels for hearing loss and blindness.


